 |
||
Ozone in the Atmosphere
Are there any other processes or methods that change the amount of ozone in the atmosphere?Natural forces can alter the amount of ozone. Remember, ozone is very unstable. It reacts easily with other atoms, and will easily donate that free oxygen atom (O1) to nitrogen gas (N2), hydrogen gas (H2), or chlorine (Cl). These atoms have always existed in the stratosphere, and they are released from a wide variety of sources (volcanoes, oceans, etc.)
Scientists have discovered that ozone levels change as part of natural cycles on Earth. Ozone levels vary slightly with seasonal changes (winter to summer as an example), wind circulation patterns, and solar cycles. If a large volcano erupts, it can send enough material into the stratosphere to increase the breakdown of ozone. Natural processes appear to have regulated the balance of ozone in
the stratosphere over the Earth’s lifetime. Ozone production = ozone
destruction. But in the early 1970s, scientists found some startling
evidence that humans were contributing to the destruction of ozone.
Many technological advances of the time included chemicals containing
chlorofluorocarbons (CFCs for short). CFCs contain a chlorine, fluorine,
and carbon atoms that are bound together. CFCs are stable. This may
be great in an appliance like a refrigerator, but this is disastrous
for CFCs that find their way up into the atmosphere. CFCs do not react
easily with other chemicals in the lower atmosphere (troposphere).
But UV light can break up a CFC, making it highly reactive. In the
lower atmosphere, CFCs are protected from UV light from the ozone
layer in the stratosphere. But as CFCs rise, they move into the stratosphere.
The UV light in the stratosphere breaks up the CFCs. They release
chlorine, and these free chlorine atoms rip oxygen atoms off of ozone,
leaving ordinary oxygen gas. Chlorine + Ozone -> chlorine monoxide +oxygen gas This would not be a huge problem, except for one thing: chlorine monoxide will collide with free oxygen atoms (O1). This oxygen atom will break apart the chlorine monoxide, releasing the chlorine atom back into the stratosphere to degrade more ozone. The chlorine atoms keep cycling through the process of breaking up ozone, and it has upset the balance of the ozone system. This reaction happens over and over again, allowing one chlorine atom to destroy many ozone molecules. ClO + O1 -> O2 + Cl Chlorine monoxide + oxygen atom -> oxygen gas + chlorine atom View Animation 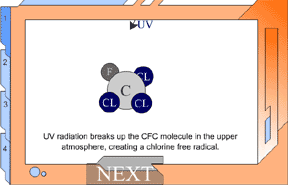
So, do chlorine atoms last forever, cycling through the stratosphere and breaking up ozone? Fortunately for us, they don’t. When a free chlorine atom reacts with other gases that contain hydrogen, the chlorine will be bound to the hydrogen to form HCl (hydrochloric acid). This can be carried into the troposphere and washed away by rain or snow. If humans then quit putting CFCs into the atmosphere, the ozone layer could eventually (in theory) repair itself. Summary of Ozone DepletionOzone depletion is a growing problem, but by banning the use of CFCs countries of the world hope to eventually restore the ozone balance to the Earth’s atmosphere. However, right now ozone loss is more than ozone produced. The most critical area seen with decreased ozone is over Antarctica. There are stratospheric cloud and ice particles that are not present in the stratosphere in warmer regions. Reactions that destroy ozone more quickly happen on the surface of these ice particles. Ozone levels drop so low, especially in the spring, that scientists call it a “hole” in the ozone layer. This allows UV light to reach life at the surface, and this light causes damage to the genetic material (DNA) of all living things who are exposed to it for long periods of time. Because of the low temperatures on Antarctica and the minimal amount of life existing there, the area of most concern is Australia. People, plants, and other animal life in the region have experienced increased rates of cancer, cataracts, etc. Could what happened in Australia get worse? Could the Earth lose its ozone shield altogether? We can only hope and pray that we have averted the problem of losing our “sun shield” in the future. It will take time to produce enough ozone to replace what has been lost. |
||

