Star Life Cycle
Fusion
As mentioned earlier in the lesson, stars generate energy by taking small Subatomic Particles and combining them to make larger particles. This process is known as fusion. The intense heat and pressure in the core of a star make it a swirling, chaotic storm of tiny particles. Because of this, the interactions between the individual particles can be very complicated. However, through careful observation of stars like our sun, astrophysicists have determined many of the ways that these reactions work.
Subatomic Particles
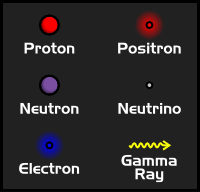 Even though astrophysicists looks at the largest objects in the universe, you can't understand how they work unless you also understand the smallest objects in the universe. These are particles that are even smaller than a single atom, and are thus called Subatomic Particles. Atoms, as you may know, are made up of a nucleus surrounded by orbiting electrons. The nucleus of an atom is made up of protons and neutrons. Besides these, there are many other subatomic particles that we need to know about in order to understand the internal workings of a star.
Even though astrophysicists looks at the largest objects in the universe, you can't understand how they work unless you also understand the smallest objects in the universe. These are particles that are even smaller than a single atom, and are thus called Subatomic Particles. Atoms, as you may know, are made up of a nucleus surrounded by orbiting electrons. The nucleus of an atom is made up of protons and neutrons. Besides these, there are many other subatomic particles that we need to know about in order to understand the internal workings of a star.
Protons are one of the fundamental building blocks that make up atoms. In fact, the simplest atom, a hydrogen atom, is just one proton orbited by a single electron. In stars, most of the hydrogen has been ionized (losing its electron), which means that when we're talking about hydrogen inside of stars, we're usually just talking about protons.
Protons are fairly large and heavy for subatomic particles, and they carry a positive charge.
Neutrons are very similar to protons, in that they are found in the nucleus of an atom, and they are fairly large. Unlike protons, however, a neutron has no charge. Neutrons are important in the creation of atoms because they help stabilize the nucleus. An atom that has too many or too few neutrons generally won't last very long, and will simply break apart into smaller atoms that are more stable.
Electrons are negatively charged particles that generally orbit the nucleus of an atom. Electrons are much smaller than protons or neutrons. Despite being so small, their charge is as strong as a proton, which means that one proton and one electron will balance each other out.
Even though electrons don't normally exist in the nucleus of an atom, the nucleus of an atom will occasionally give off an electron in a process known as Beta Decay. When this happens a neutron turns into a proton and an electron is released in order to balance out the charges. The opposite can also occur, where the nucleus of an atom can absorb an electron, changing a proton into a neutron. This is known as Electron Capture.
Positrons are particles of antimatter. They are the antimatter equivalent to an electron, which means that they are small, positively charged particles. Positrons are created during the process of hydrogen fusion, where they carry away the positive charge from protons so that they can become neutrons. However, since positrons are antimatter, they don't usually make it very far. As soon as one comes into contact with an electron (which most atoms have a lot of), the two particles annihilate each other, releasing Gamma Rays.
Neutrinos are very small, neutrally charged particles. They are even less massive than electrons and positrons. Because they are so small and they don't interact with electromagnetic fields, neutrinos usually pass straight through solid matter, making them very hard to detect. They carry energy away from reactions in the form of their own kinetic energy. Since it's very unlikely for these tiny particles to interact with any others on their way out of the star, the generally carry their energy away into space.
The only time neutrinos really react much with other particles is during huge neutrino bursts, like the kind that occur during a supernova. During a supernova, there are so many neutrinos released that they crash into other particles, transferring huge amounts of energy and starting off new fusion reactions.
Gamma Rays are photons, or particles of light, with extremely high energy. Gamma Rays have no mass, but they can carry huge amounts of energy and can still interact with other particles. That makes gamma rays one of the most dangerous kind s of radiation for humans.
The Proton-Proton Chain
In a Main Sequence star, most fusion reactions involve taking hydrogen and turning it into helium. This kind of reaction usually begins by two hydrogen nuclei, which are simply protons, crashing into each other. Since it starts with a crash between two protons, this reaction is called the Proton-Proton Chain. However, since there are a lot of different particles flying around inside a star, the Proton-Proton Chain can go a number of different ways. These different reactions are called branches.
The PPI Branch
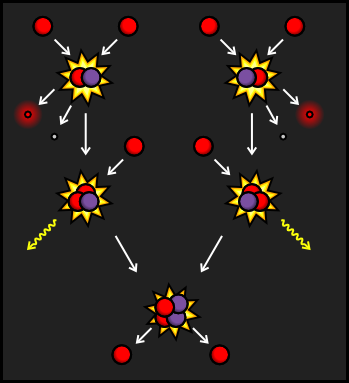 The PPI branch is the most common way that the reaction occurs in our Sun, as well as in similar-sized stars. It starts with protons colliding to create Deuterium, also known as Hydrogen-2, a hydrogen nucleus that contains both a proton and a neutron. The extra charge from the second proton is carried away by a positron and additional energy is carried away by a Neutrino. The Deuterium then collides with a third proton, leaving Helium-3 and emitting a Gamma Ray.
The PPI branch is the most common way that the reaction occurs in our Sun, as well as in similar-sized stars. It starts with protons colliding to create Deuterium, also known as Hydrogen-2, a hydrogen nucleus that contains both a proton and a neutron. The extra charge from the second proton is carried away by a positron and additional energy is carried away by a Neutrino. The Deuterium then collides with a third proton, leaving Helium-3 and emitting a Gamma Ray.
The Helium-3 nucleus then collides with a second Helium-3 that was created in the same way as the first. This creates a stable Helium-4 nucleus and frees two protons to start reactions elsewhere in the star. If we look at the input and output of this reaction, we can see that six protons go in, and a helium nucleus, two protons, two positrons, two neutrinos and two gamma rays come out.
Even though this is how most reactions occur in medium-sized stars, things sometimes go differently. With all the different kinds of particles churning about inside the star, they often combine in a slightly different sequence.
The PPII Branch
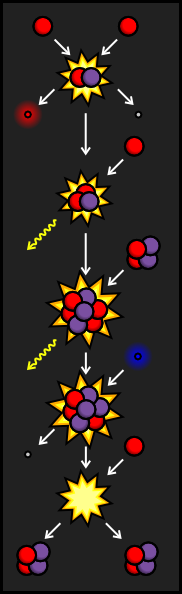 Sometimes, the Helium-3 atom from the PPI Branch crashes into a Helium-4 atom before it finds another Helium-3. When this occurs, we end up with a particle made up of four protons and three neutrons. That makes this atom Beryllium-7. This kind of Beryllium, however, is very unstable. In order to become more stable, it will try to absorb an electron, which has a negative charge. This cancels out the charge from one of the protons, transforming it into a neutron and sending out a neutrino. The resulting particle still has an atomic weight of seven, but only has a positive charge of three, making it Lithium-7.
Sometimes, the Helium-3 atom from the PPI Branch crashes into a Helium-4 atom before it finds another Helium-3. When this occurs, we end up with a particle made up of four protons and three neutrons. That makes this atom Beryllium-7. This kind of Beryllium, however, is very unstable. In order to become more stable, it will try to absorb an electron, which has a negative charge. This cancels out the charge from one of the protons, transforming it into a neutron and sending out a neutrino. The resulting particle still has an atomic weight of seven, but only has a positive charge of three, making it Lithium-7.
When Lithium-7 is struck by a proton, it creates two stable Helium-4 nuclei. Although this chain reaction is less common in our sun than the PPI Branch, it is more common in stars that burn hotter.
The PPIII Branch
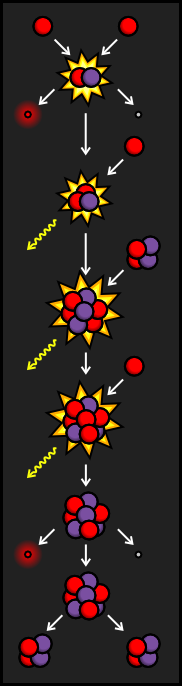 If the unstable Beryllium-7 from the PPII Branch is hit by a proton before it can find a free electron, it becomes Boron-8. Like many other particles created in fusion reactions, Boron-8 is very unstable. It soon decays into Beryllium-8 by emitting a positron and a neutrino.
If the unstable Beryllium-7 from the PPII Branch is hit by a proton before it can find a free electron, it becomes Boron-8. Like many other particles created in fusion reactions, Boron-8 is very unstable. It soon decays into Beryllium-8 by emitting a positron and a neutrino.
Beryllium-8 also happens to be very unstable. Since it has four protons and four neutrons, it splits into two very stable Helium nuclei only fractions of a second after being formed.
Like the PPII Branch, the PPIII Branch doesn't account for much of the fusion taking place in the Sun. It generally occurs at even higher temperatures than the PPII Branch, which means that very hot stars are likely to have a great deal of Helium being created by the PPIII Branch of the Proton-Proton Chain.
The CNO Cycle
In the Proton-Proton Chain, Helium nuclei are created from nothing but protons. In more massive stars, however, the protons sometimes get a little help along the way. The Carbon-Nitrogen-Oxygen Cycle, or CNO Cycle, uses carbon, nitrogen and oxygen atoms to complete the process. These helper atoms are called catalysts. As with the Proton-Proton Chain, the CNO Cycle still takes in protons and puts out helium. No new carbon, nitrogen or oxygen are created in the process. Instead, these heavier elements absorb the protons, change two of them to neutrons, then throw out stable helium nuclei (along with a bunch of neutrinos, positrons and gamma rays).
As the catalyst atoms absorb and release other particles, they transform between different types of carbon, nitrogen and oxygen. Although these catalyst atoms will fuse with protons, they don't fuse with each other at this stage because carbon burning takes much more energy than hydrogen burning.
Like the Proton-Proton Chain, the CNO Cycle has a number of different branches that the particles can take in order to get to stable Helium nuclei. Astrophysicists have discovered around seven different branches to the CNO Cycle.
Helium Burning
While a star is in the Main Sequence, most of its energy comes from Hydrogen Burning, which combines hydrogen atoms and turns them into helium, which builds up in the star's core. Helium is a very stable atom. Although Helium atoms sometimes collide with one another, the resulting atom, Beryllium-8, doesn't last very long, and will quickly decay back into two helium atoms.
When a star grows old and its core begins to collapse, the helium in the core gets hotter and more compressed. That means that the helium is moving faster in a smaller amount of space. This means that collisions between helium atoms will become more frequent. Although Beryllium-8 is still very unstable, reactions begin taking place so fast that the Beryllium will start fusing with other helium atoms before it has a chance to decay. This creates Carbon-12, another stable element.
Burning Carbon and Heavy Elements
In the most massive stars, elements heavier than helium can also be fused. When the helium in the core of such a star is depleted, it will undergo another core collapse, creating enough heat and pressure to ignite carbon burning. When carbon in the core is depleted, a core collapse will initiate Neon burning.